Wednesday, April 6, 2011
4/6/11
The first column is the number trial, the second column is the distance we walked, the thrid is the time it took to walk that distance, and the fourth is the speed in which we walked.
1 12 feet 3.19 3.76
2 12 feet 2.09 5.74
3 12 feet 3.06 3.92
4 12 feet 3.02 3.97
5 12 feet 2.67 4.94
6 12 feet 3.35 3.58
7 12 feet 2.61 4.60
8 12 feet 2.92 4.11
9 12 feet 2.80 4.29
10 12 feet 2.66 4.51
11 12 feet 3.67 3.27
12 12 feet 3.32 3.61
13 12 feet 3.38 3.55
14 12 feet 4.00 3.00
15 12 feet 3.14 3.82
16 12 feet 3.26 3.68
17 12 feet 2.94 4.08
18 12 feet 3.31 3.63
19 12 feet 3.09 3.88
20 12 feet 3.63 3.31
The average speed for our experiment was that we walked 3.96 feet per second. To make sure we had accurate results, we ran the test 20 times. If we ran the test one time our results may have been inaccurate, but now we know that our final average is an accurate number.
-JM
Tuesday, April 5, 2011
April 4th 2011

Geocentric

Heliocentric
Today in class we first continued to work on our simulations. For the group I was in, we finished discussing our answers. We had some disagreements but finally came to a conclusion. Then, Mr. Finley came over to our group to explain the next lesson. He told us to think about the following simulation. You are playing a board game in a car. Is the board game moving? Many would say no. We say no because to our eyes we see the board game staying still, but would it be moving to someone standing outside of the car? Yes, this idea is called a reference frame. What does this have to do with planets? When we look up at the moon, we see only one side. To us, we think the moon is perfectly still. This is not true. For a person standing on another part of the earth, their moon will look different then our moon. The only way we would be able to see movement of moon is if we were to stand on something other than the earth, such as the sun. Then, we would be able to see the moon move. Mr. Finley then told us more vocabulary.
Heliocentric model- Everything rotates around the sun
Geocentric model- Everything rotates around the earth
As you should know, we follow the heliocentric model. Scientists and astronomers have proven that the earth, and any other planets rotate around a sun. Then Mr. Finley told us to write a long paragraph explaining why people in history used to follow a geocentric model versus our heliocentric model? This is what we had for homework that night.
ZK
Friday, April 1, 2011
Lunar Phases!
Wednesday, March 30, 2011
March 10
After going over the homework, Connor sat on top of the cart while Josh pushed him. Josh easily pushed Connor. Then, Colleen sat on the cart next to connor. Josh had some difficulty pushing the two. Then, Kevin sat on the bottom of the cart. When Josh tried to push the cart, the cart didn't move. We concluded that mass was added each time another person was added so the cart didn't accelerate as much as when there was only one person.
Check out this website to help you understand this concept better-
http://www.thechemistryteacher.net/graphics/Sample_Activity.pdf
TR
Tuesday, March 29, 2011
Q1. Answer is A. Q2. answer is B. Q3. answer is C. Q4. answer is . Q5. answer is B. Q6. answr is A. Q7. answer is A. Q8. answer is D. Q9. answer is D. Q10. answer is A. Q11. answer is B. Q12. answer is A. We went over the homework by discussing it in our groups and then the whole class went over it.
Then we went over what we did on monday close to the end of class about how the moon controls earths tides in the ocean. Where every the moon is on the eastern or western hemosphere the tide will still be the same. When the sun and the moon are aline there is high tide. When the moon and sun aren't aline the tides are low. The moon has a greater force on earth because it is closer than the sun is.
- H.K.
Monday, March 28, 2011
Monday, March 28
After talking about what we just saw, we wrote three scenarios down.
-Jack and T.J. leaned against one another
-Finley punched a wall
-Finley pushed T.J.
We concluded that whenever you exert a force on something, it exerts the same force on you.
We then talked a bit about forces and Sir Issac Newton.
He had three laws of motion:
1. An object not moving stays not moving and an object moving continues moving at a constant rate unless an unbalanced force is exerted.
3. When things interact, they exert forces on another - equal in size and in opposite directions.
2. a=Fnet over m means the more force, the greater acceleration. More mass, slower acceleration.
After this, we got into the moon and the force it exerts on the earth. The tides are something big and controlled by the moon's force.
We drew a picture (you might now be able to read mine but I'll point them out).

The oval-like circle represents the tides, the round one the Earth. The arrow is a force diagram of the moon on Earth.
We noted that whatever happens on one side happens on the other.
I would have more, but I ran out of paper in my notebook and used a sheet of scrap paper.
-EL
Thursday, March 24, 2011
Thursday March 24
After that we did an experiment where Colleen held a laser still and Mr.Finley turned the globe around. Before the earth was turned arouned the dot was on the Tropic of Cancer. When it was turned around it was on the Tropic of Capricorn because of the tilt. This showed where the sun is in the Winter and Summer. We also comncluded that there are no seasons in between the Tropics and even if there are there very mild.
After that we did an experiment with a cicle and a bowling ball where we removed a part of the circle and the bowling ball went straight which showed that if the sun disapeared the planets would just go staight.

TJ
Wednesday, March 23, 2011
Wednesday, March 23 Period 1
- In diagram A the suns rays are hitting directly on the earth where we are not.
- on diagram B the suns rays are hitting directly where we are.
- Although the suns rays are htting above the equator where we are in A there is not
a direct path to the earth where we are so it will be winter in New Jersey.
- Also the counties near the equaotr do not experience season like we do in New Jersey. This happens because the sun always has a direct path to those parts of the earth. Since that dsent change neither does the weather.
MJ
Tuesday, March 22, 2011
March 22, 2011

Today in class we talked about our season simulation.
We discussed the biggest mistake people make. The mistake is, people think the when the earth is orbiting around the sun, and it's closest to the sun that's when it is summer and spring. But it is really the opposite.
Then we learned that our season have nothing to do with where the earth is. It depends on where the axis is tilting, and where it is facing.
We did an experiment where the table was the sun. He got a globe and circled the table. We had to determine Which way the eath was facing.
Mr.Finley then told us that our Earth has a tilt of 23.5 degrees on its Axis
-Both orbit an dtilt causes planet to be tilted towards or away from the sun.
-Because of this some parts won't have as much of a "concentraction" of rays.
At the end of class we did an experiment. We got a light and shinned it on a solar pannel. As we raised the solar pannel the number decreased.
Monday, March 21, 2011
season simulations
http://projects.astro.illinois.edu/data/Seasons/seasons.html
this is the simulation we had done!
By the way James is back yayyyy! Bye
DP
Thursday, March 17, 2011
March 17, 2011
Then, we discussed why planets orbit stars. As a class we came up with a hypothesis that a celestial object pulls a planet in if it is massive. This is why the earth obits the sun and the moon orbits the earth. After we came up with this hypothesis, we put it to the test. We set it up by using a ruler, a magnet, and a paper clip. We would inch the paper clip foward until in snapped onto the magnet. In this ccase, the sun was the magnet and the earth was the paper clip. If the earth got too close to the sun it would be sucked into it, but since we are at the perfect distance, we still get pulled in but not too much.
Now we know why the planets orbit a star. One part that tripped me up in the homework last night was that I kept trying to get a perfect circle for my planet orbit. Then I realized that it wasn't possible. The shape that all of the planets had to move in was an oval shape. When the planet gets closer and closer to the sun, it speeds up because of the unbalanced forces.
KS (4th blog)
Wednesday, March 16, 2011
March 16, 2011 - The Start of Astronomy
Well, you could describe the motion as going straight or forward. Where is it going, could be described as the same. To hit it to make it go into another direction would be to kick it on the side. One group said if you hit it directly on the top, it would not change direction.
So, hypothesis:
a) Hitting it halfway will make the direction move.
b) Hitting it anywhere can make the direction move.
c) Almost anywhere can make it change.
Now we test out Hypothesis C because most groups thought it was correct. We took TJ to test this. TJ took the long brush [ like a mallet ] to hit the bowling ball with. Look at this diagram:

The force of TJ hits the ball while it is moving. The motion then changes it direction. A new idea was made. Groups then thought that, if TJ kept hitting the bowling ball, the shape would be in a circle.

We then watched a video that shows a man hitting a bowling ball with a mallet in this kind of manner. Our hypothesis was therefore, correct.
Now we take this idea to a whole new level. How is this the same at the Earth rotating around the Sun? Look at this picture below now, too, for a better understanding-

This is a simple picture showing the Earth's rotation around the Sun. How is this similar to the bowling ball being hit by TJ? And how does the Sun make this happen?
The bowling ball is similar to this because of it's circular motion it's making. Pretend the bowling ball in the video was going around a larger ball. Imagine the bowling ball as the Earth, and the larger ball in the middle the Sun.
But how does the Sun do this? We believe it's a force the Sun is making onto the Earth...which we will investigate.
CP
Monday, March 14, 2011
3/14/11
Our simulation was on a man pushing a box across the floor. We changed the surface in which we were pushing the object on and we changed the amount of force that the man was useing to push the object across the suface.
The applied force was 28.65N, and we put the object on ice. The box moved all the way acorss the ice to the wall but it moved very slowly across it. Then we applied 569N of force and it moved much faster than before. In both tests we only pushed the object once and use 569N of force one time. When we puched the box across the surface (which was wood) and we pushed it and the object slowed down after we fush it. We had to use a greater force to puch the object across the wood. We pushed the object with 600N of force and it sped up as we push it, then we pushed the object one time with 600N of force and the object moved at a constant rate. When we kept pushing the object it was unbalanced because there is nothing pushing against it and when we pushed it one time it is balanced because there is an equal force and it moves at a constant rate.
JH
Sunday, March 13, 2011
March 11, 2011- How things slow down
We started by going over our homework.
Mr Finley put the correct answers on the board, and our goups had to talk about them.
The correct diagrams where :
DOT
a. ._.__.___.____.______.________.
b. ._.__.___.____._______.________.
c. .
d. .__________._______.____.___._..
e. ._._._._._._._._._._._._._.
FORCE
See someone else's notes.
Then Yolanda asked why E slowed down. Wouldn't the ball just stop in the hand?
The answer is no, no matter how light an object is, when your hand catches it your hands go down a little bit, depending on the weight. When an object stops, first it has to slow down. We watched this video to prove it. Only the beggining matters for what we are doing in class.
Video- http://www.youtube.com/watch?v=QfDoQwIAaXg
Then to explain this futher Mr finley threw an orange. As it goes up the orange slows down, and the top it almost stops, then it goes down speeding up.
Then we pushed a ruler across the surface of the table. After a few seconds it slowed down. This was because the surface of the table had a negative force on it. At the same time there were three different forces on the ruler. (let's say you slided it to the left) There's the table holding the ruler up going up, the pull of the Earth on the table going down, and a force from the table surface going to the right.
This slowing down right before stopping concept confuses me alittle, and the fact that force diagrams can have more than two arrows goind multiple directions is something I have to remember because it makes me think that sometime aren't solutions to things there are soloutins to. I just need to look at my notes again and then I'll understand it.
Here are some interesting websites:
http://www.physicsclassroom.com/class/1dkin/u1l5a.cfm
http://www.physicsclassroom.com/class/1dkin/u1l1e.cfm
http://www.physicsclassroom.com/Class/newtlaws/
DB(4TH)
Wednesday, March 9, 2011
Outside Car Experiment
CV
Monday, March 7, 2011
spring scale/force diagram
Mr. Finley pulled me on a cart in the hallway. There was an upward and downward force, but also a sideways force. This was un balanced situation. The prediction was the harder Mr. Finley pulls, the faster i get. The data is that the cart got faster. Mr. Finley got faster because the cart got faster (applies the same amount of force). Also the cart kept getting faster because there is no opposite force to hold back the carts forward movement. If there is nothing to stop something moving sideways, and something is still applying force, then it will just keep getting faster.
JC
Friday, March 4, 2011
3/4/11
Thursday, March 3, 2011
3/3/11!
To learn about force, Kevin had to stand with both of his arms out, with a bowling ball in one hand and a ping pong ball in the other. He obviously needed to work hard to keep the bowling ball level. To do so, he was exerting a positive force on the ball. For the ping pong ball,
he also had to exert a positive force, just not as much. He was not 'doing work' because neither the bowling ball or ping pong ball was displaced, and, since work=force x displacement, there was no work done despite how large the force is.


We then created a definition for force, which is: the interaction between 2 (or more) objects. The objects could be stuff touching it, although not directly, like with the sun, moon, earth, or even a magnet.
To learn more, we continued to study the experiment with Kevin. While Kevin was exerting an upward force, the earth was also exerting a downward force. Since these two forces were equal, the bowling ball stayed in place. If Kevin were the only thing pushing the bowling ball, the ball would simply continue upward.
Today, after reviewing last night's hw, our task was to perform a spring scale experiment. We had to measure something on the spring scale that is a good mass- not so heavy that the scale will break, but not so light that it can not be measured.
My group placed a metal circle on the scale, which measured to be 1N/100g. We created a force diagram. There was an upward arrow to represent the F of spring on mass, and a downward arrow to show the F of earth on mass, and, a circle in the middle to show the mass itself. The mass was pulling the spring scale down, while the spring scale pulled it up. It was unclear to us weather or not the forces were balanced.
 Spring Scale
Spring ScaleTo better understand the concepts we're discussing, trying some of these experiments and recording observations of them could be very beneficial.
KV (#3)
Monday, February 28, 2011
2/28/11
 the easiest constellation to see. Orion is over 1,000 lightyears away at some points.
the easiest constellation to see. Orion is over 1,000 lightyears away at some points.This is a picture of the Milky Way, it is the galaxy we live. To get from one end of the Milky way to the other, it would take 100,000 lightyears.
This simulation was very informative and it was really interesting. I have learned that Earth is extremely small when you consider the Milky Way and all of the other galaxies surrounding us. It really made me think of what else could be out there. I hope astronomers will eventually find out what else there is.
JM (#3)
Thursday, February 17, 2011
February 17th 2011
Wednesday, February 16, 2011
More Matter
Chemical and Physical Properties Examples of Physical Properties: color, size, weight, mass, texture
Finley has a cool camera thingy, and he's using it to show us what is up on the table in the front of the room.
What are some of the properties those objects have?
1) Magnet Properties
- Attract or repel other magnets
- Opposite poles of magnets attract each other
- Magnets attract some types of metals
- Above are chemical properties
- Wood will have same density no matter how big or small it is
- We can predict when water will boil. Water will boil in 100°. That is called its boiling point. Everything has its own boiling point. Not everything boils at the same temperature.
Challenge Task!
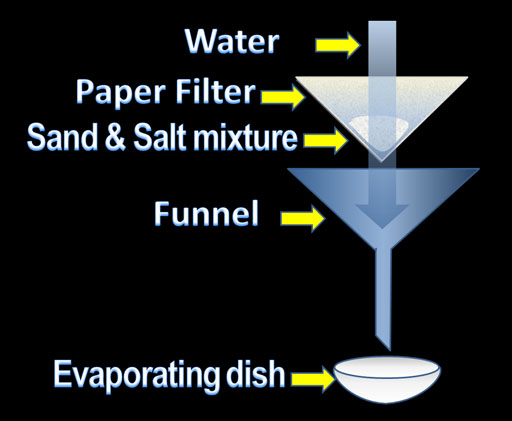
In small groups, we devised a plan on how to separate the following frozen mixture of sawdust, iron filings, sand, and sugar.
Task: Separate the frozen mixture
EXTRA CREDIT Task: determine the mass of every component of the mixture
Before we begin, we planned out all of the procedures and came up with a list of materials that we will need. We made sure to include a short paragraph that explains a justification of our procedure.
Questions: (1) What am I learning in terms of science and problem solving skills? (2) Why is it important?
.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.:.
Homework: (1) Write a procedure for the task. (2) PhET Simulation!
YZ #3
Tuesday, February 15, 2011
Code Red :(
We discussed the difference between physical change and chemical change.

Physical changes: pretty much is a change that changes its physical properties such as color, size, shape, etc. A physical change does not produce a new substance.
Ex. Ice melting, crushing a can, evaporation
Chemical changes: a change that occurs at a molecular level and produces a new substance. Usually a physical change is reversible while a chemical change usually is not. Also this process causes a rearrangement of atoms.

Ex. Burning something, cooking a egg, rusting
After going over the homework we had a code red drill so sadly I had to sit in a corner and fold origami!
YS (3)
Monday, February 14, 2011
Happy Valentine's Day!
We went over the homework. It was to work on sections of the Lab and to balance a few equations. The Lab is going to be due friday it should be typed, you would only get about half a point off if you don't.
Each table went over the balancing equations with each other. One of the equations... # 3
__Na2CO3+__HCl -->__NaCl+__H2O+ __CO2 each side has to be equal, just like an equation. the answers in red are the answers. our test will be on thursday!!!!
What can we do with our starting measurments and ending?
compare them H2O left the test tube...if you start with 57 g and end up with 23 g...34 g left the test tube. this can be good data and observations for your lab.
How does this apply to the conservation of mass?- the particles still weigh the same but are not in the same spot and the mass of the atoms went away from the test tube but if we colected them it would still be the same.
conservation of mass- mass cant just disapear what ever we start with we have to end with.
What happened to the arrangement of atoms?- everything was separate in the beginnning, all one big group the molicules were no longer together in the end, we changed the organizaton of the particles.
wich of these involved rearrangment of particles?
crumpled paper
burning magnesium ribbon
lighting a match
melting ice
soaking water up with a paper towel
nail getting rusty
the red ones were rearranged because theybecame two separate things.
in each of the RED ones the particles leave the source. unlike the Non Red ones.
GP
Thursday, February 10, 2011
February 10, 2011
Monday, February 7, 2011
February 7, 2011

Today in class, we went over the homework from the weekend and did an activity that involved lighting paper on fire.
Thursday, February 3, 2011
Febuary 3, 2011
We started looking at our simulation from homework. One example was a cup of coffee that had a little bit of particles. When the cup was hot the particles would speed up. When it was cooler, they slowed down. They all bounced off each other.
In our groups we discussed how a thermometer works. Mr. Finley asked us if you put a cup of coffee in the microwave and took it out then put a thermometer in it, what would happen? We learned that the energy of the hot water transfers to the glass particles which transfers its energy to the red stuff in the thermometer. Thermometer measures kinetic energy.
-FC
Monday, January 31, 2011
Matter


By, Danielle Pagano
3rd blog
Wednesday, January 26, 2011
January 26, 2011
 eded to put a water bottle in the freezer and leave one water bottle at room temperature and wait for a few hours. After 3 hours we would check the results. We noticed that the room temperature bottle kept its shape and stayed normal, but the freezer bottle was a different story. The freezer bottle seemed to colapse on itself. This happend because there were more air particles in the freezer outside the bottle than there were inside the bottle. These outside particles had a stronger force than the inside particles causing the water bottle to look like the picture on the right. (Freezer bottle on the left, room remperature on the right)
eded to put a water bottle in the freezer and leave one water bottle at room temperature and wait for a few hours. After 3 hours we would check the results. We noticed that the room temperature bottle kept its shape and stayed normal, but the freezer bottle was a different story. The freezer bottle seemed to colapse on itself. This happend because there were more air particles in the freezer outside the bottle than there were inside the bottle. These outside particles had a stronger force than the inside particles causing the water bottle to look like the picture on the right. (Freezer bottle on the left, room remperature on the right)Tuesday, January 25, 2011
January 24, 2011

We also talked about how to find the density of an object and the formula is Density = Mass / Volume.
Next we went on the Phet website to do a simulation on density. There was a few pages we had to fill out for classwork. In the simulation we were told the mass of an object in kilograms. Then we found out the volume in liters by putting the block in water to see how much it displaced the water and that was the volume. And to find the density we took the mass and divided it by Liters or the volume. We also had to determine if the block sunk or floated and if so how much. Water has a density of 1 kg / Liter and any block that was greater than that sunk because the density was greater than what water could hold up. If the amount of kg / L was less than that of water it floated. For example if the block had a density of .6 kg / L, the block would float and since that is less than the density of water. So the block would floating, but 60 percent of the block would be under water. I got 60 percent because .6 kg is 60% of the density of water which is one kg per Liter. Finally, what ever part of the pages we didn't finish was to be done for homework.
KM
Thursday, January 20, 2011
Packet 1- 19- 11
In the packet, we had to perform an experiment. We had to find two different ways to find the volume of the following objects: a dice, a coin, a transformer, and a ping pong ball. The two ways my group chose were to use a ruler to measure the object, or use a beaker. First, we would fill the beaker with about fifty milliliters of water. Then, we would drop the object in the water, and measure. Then we would subtract the new measurement by the old one to see the volume. The volume for the dice was 3 ml. The volume for the coin was 0.5 ml. The volume for the transformer was 24 ml. The volume of the ping pong ball is 20 ml.
The last couple of questions were about finding the volume of different objects such as, a bottle of soda, an ipod touch, an Xbox 360, and a bottle of cough syrup. Also, there was a question on how many minutes are in an hour, and how many hours are in a minute. There are sixty minutes in an hour and 0.06666666667 hours in a minute Mr. Finley wants this packet to be turned in.
This website explains how you can use a beaker to find volume:http://www.schools.utah.gov/curr/science/core/7thgrd/sciber7/matter/html/VOLUME.HTM
TR
Voume, Mass, and Density
Volume Mass
Most evacuted 1000g 900g
Open to air 1000g 1000g
Pumped with 1000g 1100g
air
a) What happened to the volume of the container when to you filled it with air?
The volume stayed the same because the measurements of the container stayed the same.
b) What happened to the mass of the container, when you filled it with air.
It became heavier because you added more air particles.
Then we drew particles pictures of each situation, Most Evacuated, Open to Air, and Pumped up with Air.
We then did a quick experiment like the one we did in our homework once with a balloon in a vacuum. This time though we used a glove, and when we sucked the air out of the container with the glove in it, the glove expanded. There wwere no air particles blocking it from doing so.
We then went and did two problems.
Problem:
You have two identical beakers. one has 90ml of salt water, which has a mass of 100g. The other has 100 ml of tap water, which has a mass 100 g.
a) Make a particle picture of both liquids.
b) Which is denser? How do you know?
Reason
You have a pice of aluminum that has a mass of 21.6 g. The aluminum rectangle has a height of 1cm, a width of 2 cm and a length of 4 cm. Other piece of aluminum with a mass of 32.4 g has a height of 2cm, a width of 2 cm and a length of 3 cm,
a) Make a particle picture of both pieces. Are they the same or different?
b) What is r=the volue of each block?
c) If the mass of the particles is 21.6g for 8cm cubed what is the mass of the particles in each centimeter of the aluminum?
For the rest of the of the problem check our hw for tonight.
We also defined density.
Density- the amount of particles in the same amount of space
The one that that still cinfuses me is the density part. I don't have an idea for a unit rate or radio. I can see that mass Density and volume are related, I'm just not sure how.
Videos-
Balloon in vacuum: http://www.youtube.com/watch?v=VoiimTycDy8&feature=related
Volume and Density: http://www.youtube.com/watch?v=rxb
DB (3rd Round)
Monday, January 10, 2011
Then Mr. Finley, continuing his destructive rampage decided to add sodium bicarbinite to water in a almost airtight bottle that was extremly thin but somewhat tall and it blew up. Then he put it in a big ziplock bag with the same exact volume and it did not blow up do to the fact it has space to expand. The following is a link to a similiar experiment with sodium bicarbonite.
http://www.Youtube.com/watch?v=wiZVC6P8vmU
CV
Thursday, January 6, 2011
Last night's homework: 1. consider the experiment: You pour 10 ml of salt into 100 ml of water. Today we are working on an experiment that is similar to the one from last night.
Today's experiment: We pour 10 ml of suger into 100 ml of water.
Someone had an idea that the water had more open space than a gas.
Based on that some predictions said: That the water should raise and the suger should dissolve.
Question: did 100 ml of water + 10 ml of suger = 110 ml?
Results: The total volume was not 110 ml. The suger dissolve and it went into the empty space of the water.
AR
Wednesday, January 5, 2011
Air Particles
Then, we did an experiment, Mr. Finley put some sort of smelling alcohol on a chair and had about 10 people stand around it. We said that the people in the front would smell it first and the people in the back would smell it last. The first two people to smell it were katie and i. then TJ and Anderson, then it went around the circle. Not everyone smelled it at the same time, and the particals moved at all different speeds. So they may have reached one person before they got to another person. In the end everyone smelled it except for connor. Particals move in random directions, they move at random speeds, and they move in all directions. When particals get light shun on them they get something like and energy boost and they speed up and go off in random directions.
JH
Tuesday, January 4, 2011
We our watching a video on how the air is obsorbing the liquid. This guy put a wet piece of paper in a glass contanier with no air and the other just out in the paper. In the end, the one inside of the glass contanier dryed up faster. So what this means is that the air is soaking the alcohol.
Now, the paper went back to it's starting point which is 2.7. This means that the alcohol left slowly, not fast. We know this because each time we checked the mass was going down by either one or two grams. We need to figure out how it was going down slowly. Jack said that it was made up of different things. The smaller parts go first and the bigger go last. They also said that the bigger spots have different parts inside of the alcohol which means that it would take much longer to go.
KQ
Monday, January 3, 2011
Monday, January 3
Mechanism Test Prediction
Table soaked it Repeat without paper Water stays on paper
Paper soaked it *Left blank* *Left blank*
Air took it Create a vacuum chamber Water stays on paper
Light soaked it Enter a room without light Water stays on paper
Prediction - a guess of the outcome
Vacuum - container where air (or other) can be soaked up
The only mechanism we were able to test was the first one. We repeated the feat, except without the table that time. (We hung the paper onto something that looked like a piece of rope on the ceiling.)
-EL


